
Virtual Screening
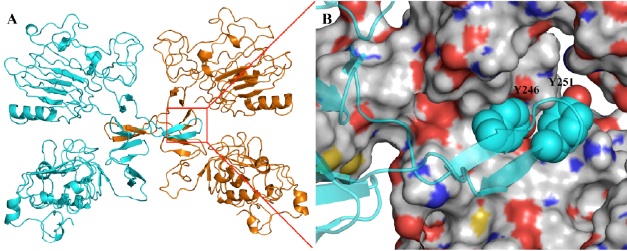
Using a crystal structure of apotential therapeutic target, a docking program screens a virtual library of compounds to identify the bound-ligand conformations and estimated affinities prior to purchase and experimental binding assays. In the case illustrated, the crystal structure of the activated EGFR dimer is shown (A) with beta-hairpin dimerization arm bound in the recognition site. In B is shown the two tyrosine residues of the dimerization arm that comprise a significant aspect of recognition.
Using the recognition site as a target for virtual screening, several drug-like molecules were identified and shown to function by inhibition of dimerization. (see Yang et al. Chem Biol Drug Design, 76:1, 2010)
A design and synthetic project involves peptidomimetics of the beta-hairpin dimerization arm to inhibit EGFR receptor activation (Mr. Daniel Lee, BME undergraduate).